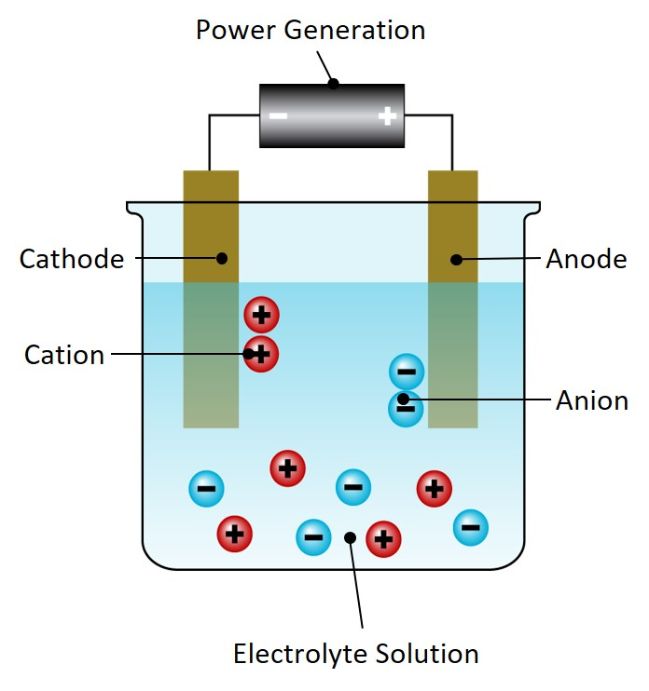
Electrochemistry involves the relationship between electricity and chemical reaction. The electrochemical cell is where the electrochemical reaction or electrolysis occurs and it consists of two conductive electrodes (the anode and the cathode) linked by an electrical circuit.
Depending on the type of electrochemical cell and desired process, the anode and cathode may be separated by a porous diaphragm or ion exchange membrane or may be undivided. Depending on the chemical reaction that takes place within the cell (oxidation or reduction), different products with multiple different applications can be produced, such as chlorine, hydrogen, oxygen, caustic soda and metals.
