De Nora On-Site Generation
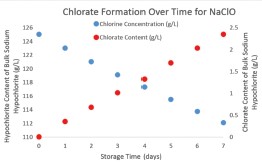
With nearly a century of electrolysis expertise, De Nora intimately understands many of the factors involved in the production of chlorate during sodium chloride brine electrolysis, including storage time, storage temperature, and hypochlorite concentration. De Nora has developed technology to control and influence these factors.
De Nora currently offers multiple on-site generation systems that address some of the concerns around chlorate formation:
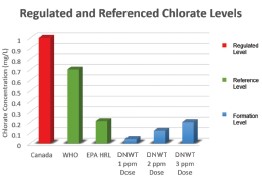
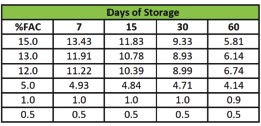
Chlorate formation from hypochlorite solutions generated by De Nora electrolysis is well below proposed regulatory levels. Chlorate is expected to be regulated by the EPA, likely at or near the international WHO guideline of 0.7 mg/L (700 µg/L). Extensive testing on the chlorate production by De Nora EC systems has been undertaken both internally and in conjunction with third party studies. Typically, the De Nora systems produce chlorate at a rate of less than 40 micrograms (0.04 mg) per milligram of free available chlorine (FAC).
Therefore, even at a high FAC dose of 5 mg/L, the expected chlorate concentration in the treated water will be less than 200 µg/L (0.2 mg/L), well below the likely 700 µg/L limit and is even below the EPA health reference limit of 210 µg/L. Typically, water treatment plants only dose 2 - 3 mg/L as free available chlorine, and at these dose points, chlorate content in the finished water will likely be half of the lowest contem-plated chlorate regulatory limit.
Since the hypochlorite solution is generated on-site, as needed, storage time is typically 24 hours or less, minimizing hypochlorite degradation.
The hypochlorite solution is generated at less than 1%chlorine concentration, which also reduces the chorine degradation rate.
Now, De Nora – including MIOX, which they acquired in early 2019 - have been working independently for several years to better understand how chlorate is produced during electrolysis. De Nora is actively conducting research at multiple laboratories to understand the mechanisms resulting in the production of chlorate during electrolysis to ensure their products will be able to meet future chlorate regulatory requirements. Several new patented technologies are expected to result from these research efforts, ensuring that De Nora Electrochlorination systems will be able to meet even the strictest chlorate limitations as regulations continue to evolve.
While it’s impossible to know exactly if, when, and how low EPA might regulate the production of chlorates in drinking water, a company committed to electrolysis for more than 95 years is certain to be at the forefront of new technologies to meet and exceed these future regulations.